First published: September 28, 2020
In peptide manufacturing, solid-phase peptide synthesis (SPPS) revolutionized the manner which nearly all peptide APIs are produced. The strategy originally developed by Bruce Merrifield employs an inert solid-phase resin to immobilize the C-terminal amino acid in a peptide chain eliminating its reactivity during the successive cycles of chain elongation (1). Thus, by employing an amino protecting group and semi-permanent protecting groups on the side chain functionalities, a peptide can be assembled in a vectoral manner proceeding from the C- to the N terminus. Strategies evolved over the past 40 which utilized either a Boc-Bzl or an Fmoc-tBu strategy. The former using a strong acid such as TFA to cleave the Boc protecting group and the later using a base to remove the Fmoc protecting group. Fmoc strategies ultimately became the favored method due to the elimination of the cumbersome and dangerous HF-based cleavage required to cleave and deprotect peptides using a Boc-Bzl strategy and the special Kel-F and Teflon equipment necessary for safely handling liquid HF. Typical acidolytic cleavage for peptide synthesized using Fmoc-tBu strategy can be performed in standard glass reaction vessels and are easily scalable.
Solution phase methods are particularly slow when compared to SPPS. The economic benefits of solution chemistry are in the reduction in chemical waste generated as well as smaller quantities of the key amino acid derivatives since they are used only in very small molar excess due to the homogenous nature of the reactions. Additionally, specially protected derivatives with hydrogen labile protecting groups (Z-based) are regularly used. Hydrogenation to remove these protecting groups also presents a considerable hazard to commercial manufacturing. Usually, the C-terminal amino acid is protected as an acid labile ester. Following a coupling, a series of organic and aqueous extractions are necessary to remove reagents and excess reactants, before proceeding to the next step which is removal of the Z protecting group. This results in a slow and cumbersome process which is only practical at very large commercial scale.
Thus, an ideal solution to this would be to develop special chemistry which makes solution phase behave very similarly to SPPS. If this were indeed possible, then it may be practical to use Fmoc-tBu based amino acid derivatives in a simple solution phase approach. The benefits here are economical usage of the standard Fmoc-tBu amino acid derivatives that are readily available at extremely reasonable costs coupled with the lower waste generation of a solution-phase process. There have emerged two similar technologies that make this now practical. They both rely on a special C-terminal protecting group that eliminates reactivity at this position while simultaneously imparting unique solubility properties to the protected peptide which facilitate its ability to stay in solution in the organic solvent based coupling and deprotection steps, but allow for convenient extraction or precipitation versus aqueous wash solutions.
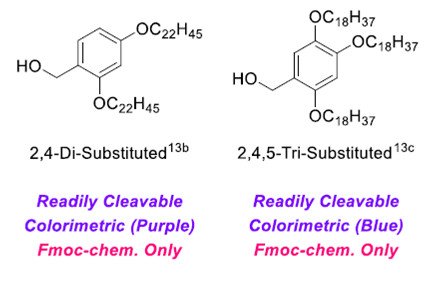
The process being developed by the Japanese company Jitsubo uses a C-terminally esterified protecting group which has a central benzyl alcohol moiety with two long alkyl side chains to impart insolubility in acetonitrile (shown above). They have named their process Molecular Hiving. The process solution chemistry steps have been developed in special green solvents such as 2 Me-THF as replacements for DMF and NMP. At the conclusion of the reaction, the product is precipitated into a MeCN. The precipitated product is subsequently washed with MeCN to remove reagents and reactants. Unreacted amino acid is removed by treatment with propylamine. The precipitated product is subsequently redissolved in Me-THF for continuation of the next cycle. (2)
A second similar technology utilizes a C-terminally esterified linker which is conjugated to a diphenylphoshine benzyl alcohol which has been named Group Assisted Purification (GAP) reagent (shown below). This technology is being developed by the USA-based company, GAP Peptides. Extension of the technology by incorporating standard linkers commonly used in Fmoc-tBu SPPS have been added to this GAP reagent allowing easier access to peptide acids and amides (3). The chemistry allows for a homogeneous solution phase process which can utilize precipitation/extraction steps to remove reagents and reactants. This process also allows for easy adaptation for Fmoc-tBu chemistry to a solution process. Conventional reactors that incorporate separation of phases to automate the process. The organic layer retains more than 99% of the growing peptide.

Each of these technologies appear to be useful for shorter sequences up to 10 -12 residues making them ideal for short peptide APIs and also for cosmetic peptides that have requirements for use of non-CMR (mutagenic, carcinogenic or reprogenic) solvents.
References
(1) Merrifield, R.B. (1963) Journal of the American Chemical Society, 85 (14): 2149–2154
(2) Okada. Y. et al. (2019) Org. Process Res. Dev. 23, 2576−2581
(3) Siefert, C.W. et al., (2016) Eur. J. Org. Chem. 10.1002/ejoc.201600026
For further reading, check out our Green Peptide Chemistry Page.

 中文
中文