First published: June 8, 2021
In May, the US FDA approved the NCE peptide drug, EMPAVELI™ (pegcetacoplan), from Apellis Pharmaceuticals, Inc., for the treatment of adults with Paroxysmal Nocturnal Hemoglobinuria (PNH). As a part of the innate immune system, the complement system helps induce responses to invading pathogens, triggering the “complement cascade” of events, with complement protein C3 at the center of the coordination of events. PNH is a rare, acquired complement disorder and has not seen a drug approval in 15 years. As a disease of the blood, it can have life-threatening effects, including hemolysis, or destruction of red blood cells, that can lead to anemia, renal failure, thrombotic and hemorrhagic events, to name a few. PNH is similar to aplastic anemia and likely affects 5,000-6,000 US adults and has a 29% 10-year mortality rate.1,2 While other existing treatments inhibit C5, pegcetacoplan targets C3 and increases hemoglobin therefore reducing the need for blood transfusions.3
Pegcetacoplan is also currently involved in several additional clinical trials as potential treatments for other complement-mediated diseases involved in nephrology, ophthalmology, hematology, and neurology. Pegcetacoplan contains two cyclic peptide C3 binding peptides separated through a sidechain linkage to a bidentate 40 kD polyethyleneglycol (PEG) polymer as shown above in Fig. 1.4,5
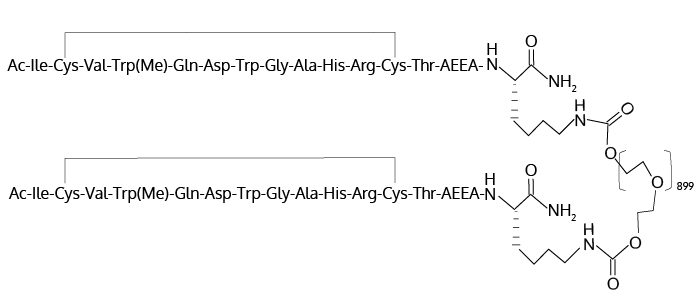
O,O’-Bis((S2,S12-Cyclo(N-Acetyl-L-Isoleucyl-L-Cysteinyl-L-Valyl-1-Methyl-L-Tryptophyl-L-Glutaminyl-L-Alpha-Aspartyl-L-Tryptophylglycyl-L-Alanyl-L-Histidyl-L-Arginyl-L-Cysteinyl-L-Threonyl-2-(2-(2-Aminoethoxy)Ethoxy)Acetyl-L-Lysinamide))-N6.15-Carbonyl)Polyethylene Glycol (N = 800-1100)
References:
- Bektas M, Copley-Merriman C, Khan S, Sarda SP, Shammo JM. Paroxysmal nocturnal hemoglobinuria: patient journey and burden of disease. J Manag Care Spec Pharm. 2020 Dec; 26(12-b Suppl):S8-S14. doi: 10.18553/jmcp.2020.26.12-b.s8. PMID: 33356781. https://www.jmcp.org/doi/pdf/10.18553/jmcp.2020.26.12-b.s3
- https://www.hopkinsmedicine.org/kimmel_cancer_center/types_cancer/paroxysmal_nocturnal_hemoglobinuria_PNH.html
- https://investors.apellis.com/news-releases/news-release-details/apellis-announces-us-food-and-drug-administration-fda-approval
- https://gsrs.ncats.nih.gov/app/substance/9ad6e782
- https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/215014s000lbl.pdf
Additional Resource:
The Complement System, https://www.youtube.com/watch?v=Zb9S_K8h1F8

 中文
中文