First published: December 6, 2019
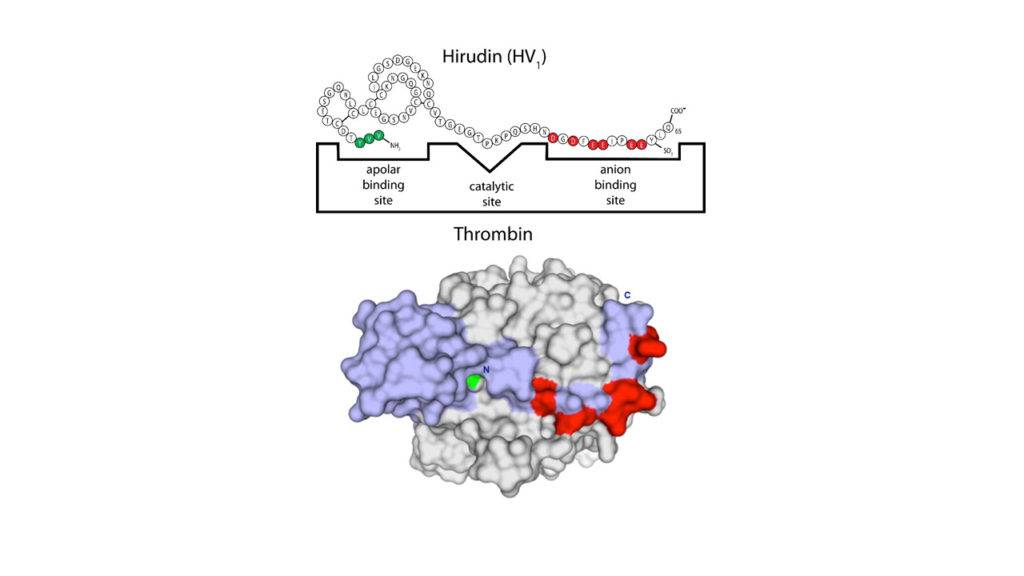
Leech therapy from the past utilized leeches to draw blood from patients. Hirudin, a peptide that is isolated from the saliva of leeches, is responsible for the anti-coagulating effects allowing leeches to continue to drain blood without it coagulating. Hirudin is a 65 residue peptide (MW=7074) stabilized by three disulfide bonds. It has an extremely anionic C-terminal region including a sulfated Tyrosine residue and a non-polar aromatic N-terminal region. This helps position the key thrombin interaction residues (Pro-Arg-Pro) directly at the active site of the enzyme. Hirudin is a direct inhibitor of thrombin (IC50= 0.92 mg/ml or 1.3 x10-7 M) which is one of the key enzymes in the blood clotting cascade. As shown in the figure above, hirudin only binds to activated thrombin and thereby prevents fibrinogen activation leading to the formation of a clot. Isolation from natural sources is not practical and lead to the development of mimetics as well as a now discontinued recombinant product (lepirudin, desulfated hirudin 1-65).
Bivalirudin, shown below, is a synthetic peptide mimetic that was developed based upon the key binding residues in hirudin. The designed peptide exploits the key anionic region and the protease blocking region (Pro-Arg-Pro) tethered together with a tetra-Gly linker to position this precisely at the active site of the enzyme. Additionally, the N-terminal Phe residue is in the D-configuration which helps prevent proteolysis of the peptide. The IC50 of bivalirudin is 12.3 ug/ml for activated thrombin.

As such, bivalirudin is an approved injectable peptide for the treatment of the following disorders:
US (United States) Indications:
- Bivalirudin is indicated for use as an anticoagulant in patients with unstable angina undergoing percutaneous transluminal coronary angioplasty (PTCA).
- Bivalirudin with provisional use of glycoprotein IIb/IIIa inhibitor (GPI) is indicated for use as an anticoagulant in patients undergoing percutaneous coronary intervention (PCI).
- Bivalirudin is indicated for patients with, or at risk of HIT/HITTS undergoing PCI.
- Bivalirudin is intended for use with aspirin and has been studied only in patients receiving concomitant aspirin
EU (European) indications:
- Percutaneous Coronary Intervention (PCI), including patients with ST-segment elevation myocardial infarction (STEMI) undergoing primary PCI.
- Bivalirudin is also indicated for the treatment of adult patients with unstable angina/non-ST segment elevation myocardial infarction (UA/NSTEMI) planned for urgent or early intervention.
- Bivalirudin should be administered with aspirin and clopidogrel.
Bivalirudin is an approved generic peptide that is produced by Ambiopharm in our cGMP, FDA-inspected facilities. Please contact us if you would like information about our cGMP-grade bivalirudin.
Note: This post is informational and not intended as medical advice, please consult a doctor before trying any treatment.

 中文
中文