First published: December 2, 2020
As we enter into the final month of 2020, we reflect back on what is probably one of the strangest years in our lifetime. The effect of the Covid-19 virus has been far-reaching and devastating to many of the minority populations and elderly in our country. The current US Federal Government has more or less given up and is now embracing the idea of herd immunity as their response to this crisis. Luckily, science has been providing a better option to produce a vaccine in record time through Operation Warp Speed. It is possible that primary healthcare workers and high-risk individuals may be eligible for the vaccine after its initial roll-out in Dec. 2020 or January 2021 following FDA approval. It is projected to be available to average citizens in Q2-2021. Ambiopharm has also been engaged in several peptide applications to potential therapeutics and vaccine candidates with our partners to aid in this Covid-19 quest as well.
Currently, there are three major vaccine programs which have recently completed a widespread phase III clinical trial. These vaccines are all quite different in their approach to develop an immune response in the patients. The programs are being developed by Astra-Zeneca, Pfizer-BioNTech and Modera. A brief description of each program follows:
Astra Zeneca AZD1222
AZD1222 was co-invented by the University of Oxford and its spin-out company, Vaccitech. It uses a replication-deficient chimpanzee viral vector based on a weakened version of a common cold virus (adenovirus) that causes infections in chimpanzees and contains the genetic material of the SARS-CoV-2 virus spike protein. After vaccination, the surface spike protein is produced, priming the immune system to attack the SARS-CoV-2 virus if it later infects the body. This viral vector-based vaccine was reported to be effective in preventing Covid infection with >60% efficacy. There were some issues reported with the manner in which patients were dosed with the vaccine that show a small subset of patients who received a half initial dose followed by a full dose were >90% effective. This issue is complicating the interpretation of the data which consisted of 27,000 patients in a double blind study. Storage conditions for this vaccine are simple requiring only refrigeration (5 C). (1)
Moderna mRNA1273
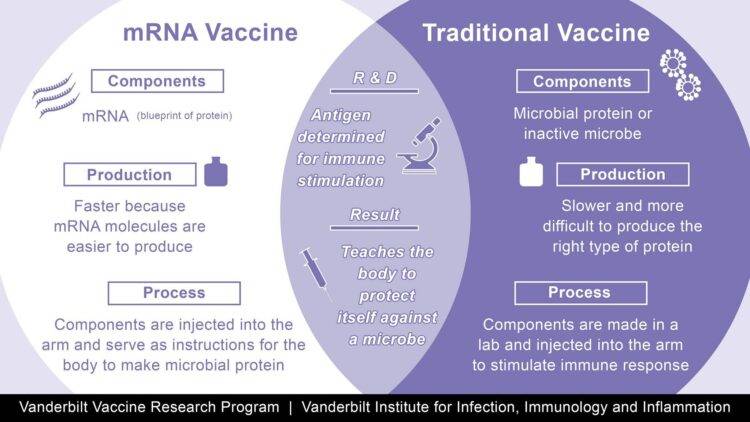
Messenger RNA is genetic material that tells cells how to make proteins. So Moderna’s coronavirus vaccine candidate works by injecting a small piece of mRNA from the coronavirus that codes for the virus’ surface spike protein. This protein helps the coronavirus attach to and invade cells, and it’s what antibodies target and neutralize. Moderna’s mRNA vaccine spurs the body to produce the spike protein internally. That, in turn, triggers an immune response.(2) Moderna conducted a phase III trial double blind trial in 30,000 patients. The Phase 3 COVE trial is a randomized, 1:1 placebo-controlled study testing mRNA-1273 at the 100 µg dose level in 30,000 participants in the U.S., ages 18 and older. The primary endpoint is the prevention of symptomatic COVID-19 disease. Key secondary endpoints include prevention of severe COVID-19 disease and prevention of infection by SARS-CoV-2. Moderna announced on Nov. 16, 2020, that the independent, NIH-appointed Data Safety Monitoring Board (DSMB) for the Phase 3 study of mRNA-1273, its vaccine candidate against COVID-19, has informed Moderna that the trial has met the statistical criteria pre-specified in the study protocol for efficacy, with a vaccine efficacy of 94.5%. This vaccine can be stored at -5 C, making its distribution relatively simple in standard doctor’s offices, clinics and pharmacies.
Pfizer-BioNTech BNT162b2
Similar to the Moderna mRNA vaccine described above, the Pfizer-BioNTech is also a mRNA-based vaccine to the surface Spike protein of the Covid-19 virus. On Nov. 18, Pfizer and BioNTech announced that, after conducting the final efficacy analysis in their ongoing Phase 3 study, their mRNA-based COVID-19 vaccine candidate, BNT162b2, met all of the study’s primary efficacy endpoints. Analysis of the data indicates a vaccine efficacy rate of 95% (p<0.0001) in participants (43,000) without prior SARS-CoV-2 infection (first primary objective) and also in participants with and without prior SARS-CoV-2 infection (second primary objective), in each case measured from 7 days after the second dose. The first primary objective analysis is based on 170 cases of COVID-19, as specified in the study protocol, of which 162 cases of COVID-19 were observed in the placebo group versus 8 cases in the BNT162b2 group. Efficacy was consistent across age, gender, race and ethnicity demographics. The observed efficacy in adults over 65 years of age was over 94%.(3) Storage of this vaccine requires extreme cold at -80 C making distribution more complicated. Furthermore, the vials must be administered within 1 hour of thawing. These issues will complicate the distribution of this vaccine.
MMR Vaccine and Covid-19
Published epidemiological data suggests a correlation between patients who receive measles-rubella containing vaccines such as the commonly available MMR vaccine, and reduced COVID-19 death rate. Similar observations were recently noted in a Cambridge Study by Young et al, who noted protein homology between the COVID-19 virus and the rubella virus, corroborating the evidence in this report. The epidemiologic associations suggest that a measles-rubella containing vaccine, as currently produced, may be protective against severe disease and death from COVID-19 exposure. (4)
References
- https://www.astrazeneca.com/media-centre/press-releases/2020/azd1222hlr.html
- https://www.businessinsider.com/moderna-designed-coronavirus-vaccine-in-2-days-2020-11
- https://www.pfizer.com/news/press-release/press-release-detail/pfizer-and-biontech-conclude-phase-3-study-covid-19-vaccine
- Gold, J.E. Tilley, J.P. and Baumgartl, W.H. (2020) MMR Vaccine Appears to Confer Strong Protection from COVID-19: Few Deaths from SARS-CoV-2 in Highly Vaccinated Populations. 10.13140/RG.2.2.32128.25607
image source: https://www.vumc.org/viiii/spotlight/how-does-mrna-vaccine-compare-traditional-vaccine

 中文
中文