First published: May 4, 2021
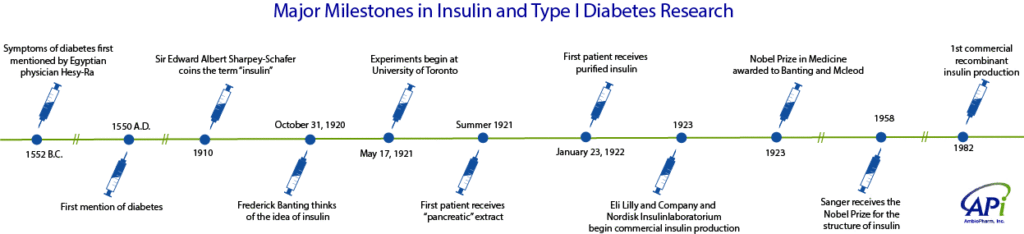
This summer marks 100 years since the discovery of insulin, when Frederick Banting began experiments on May 17, 1921 at the University of Toronto1. As shown in the timeline above, the identification of the disease and development of treatments for diabetes spans for more than 3500 years since it was first described in ancient Egypt. Up until the first doses of insulin were given, type 1 diabetes was a death sentence for those afflicted. Type I diabetes was previously called insulin-dependent or juvenile diabetes because its onset is predominantly seen in children and young adults. It is an auto-immune condition where the body’s immune system attacks its insulin-producing cells, resulting in elevated blood sugar, or hyperglycemia. Left untreated, a patient will become seriously ill and the condition is fatal.
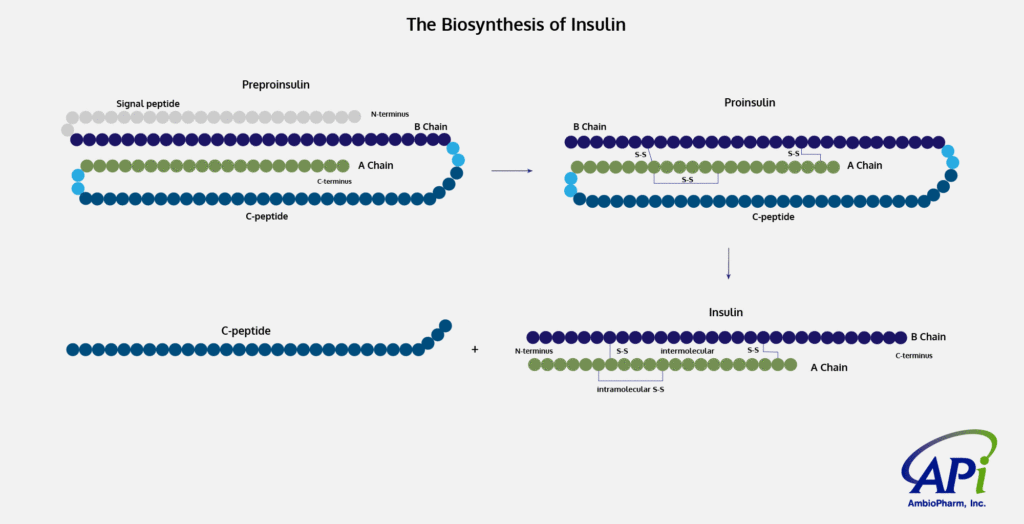
With the discovery and administration of insulin and the development of blood sugar monitoring, diabetes has become manageable. When sugar, or a source of sugar like carbohydrates, is consumed, the body releases insulin which stimulates the metabolism of the sugar. Insulin is a polypeptide hormone composed two peptide chains (the A and B chain) that are connected by two intermolecular disulfide bonds and a third intramolecular disulfide bond located in the A chain. It is produced in the beta cells2 in the islets of Langerhans in the pancreas and was the first chemically synthesized protein. It is normally produced at ratios sufficient to metabolize the glucose intake, however, when the levels of glucose in the blood (or blood sugar) are elevated, several events are triggered, including ketoacidosis which can lead to a coma. Ketoacidosis is the result of an absence of sufficient insulin, so the body turns to fats to produce glucose through gluconeogenesis which results in a buildup of ketones.3 While hypoglycemia (or low blood sugar, from too much insulin and not enough glucose) if left untreated can cause seizures and unconsciousness.4
Unlike type I diabetes, type 2 diabetes is a condition where the body produces insulin at insufficient levels, usually brought on from diet and lifestyle habits. Several glucagon-like peptide 1 (GLP-1) agonists have been developed and approved for the treatment of type-2 diabetes (exenatide, lixisenatide, and liraglutide) via daily subcutaneous (sc) injection. These function by stimulating the production of insulin in the patient following administration. More recently, dulaglutide and semaglutide have been developed which are also glucagon-like peptide-1 as weekly sc injections. As a major milestone, oral semaglutide was first approved in 20195 for type 2 diabetes and is a significant achievement in oral peptide drug delivery for diabetes treatment. This gives hope that one day scientists can develop an oral insulin for type 1 diabetes, compared to the current injectable options. Other advancements in insulin include extended action (helpful for preventing overnight hypoglycemia), improved stability, and easier use. Additionally, Xeris Pharmaceuticals6 and Zealand Pharma7 recently had rescue glucagon treatments approved (glucagon and dasiglucagon, respectively), which are much needed for diabetic hypoglycemic (low blood sugar) events. Glucagon triggers the liver to convert stored glycogen to glucose. The future looks sweet for diabetes discovery.
References:
- https://insulin100.utoronto.ca/
- https://www.cdc.gov/diabetes/basics/what-is-type-1-diabetes.html
- https://www.diabetes.org/healthy-living/medication-treatments/blood-glucose-testing-and-control/hyperglycemia
- https://www.niddk.nih.gov/health-information/diabetes/overview/preventing-problems/low-blood-glucose-hypoglycemia
- https://www.ajmc.com/view/efficacy-of-oral-semaglutide-the-pioneer-clinical-trial-program-and-implications-for-managed-care
- https://xerispharma.gcs-web.com/news-releases/news-release-details/xeris-pharmaceuticals-receives-us-fda-approval-gvoketm-glucagon
- http://www.globenewswire.com/news-release/2021/03/22/2197267/0/en/Zealand-Pharma-Announces-FDA-Approval-of-Zegalogue-dasiglucagon-injection-for-the-Treatment-of-Severe-Hypoglycemia-in-People-with-Diabetes.html

 中文
中文