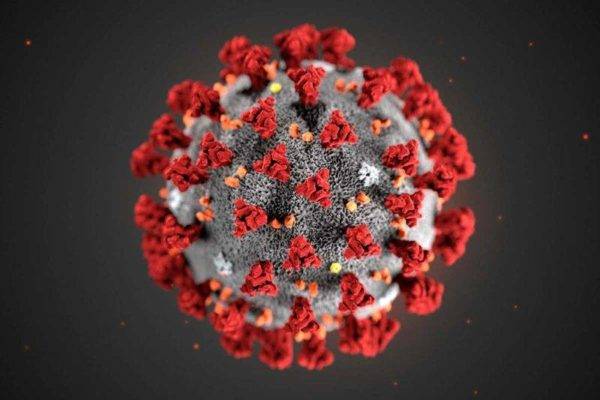
First published: March 20, 2020
With the current pandemic recently declared by the WHO with regards to the Covid-19 virus, scientists are beginning to gain insight into potential therapeutic means to treat the infection and help to ameliorate symptoms while allowing the body to mount an immune response. Ultimately, a vaccine will be useful for the general population assuming that the virus does not mutate requiring a seasonal vaccine similar to those currently produced for influenza.
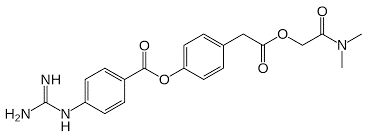
In a very recent paper published in the journal Cell (1), cellular entry of coronaviruses requires binding of the viral spike (S) proteins to specific cellular receptors and also on S protein priming by host cell proteases. The SARS-CoV-2 (aka Covid-19) utilizes the SARS-CoV receptor Angiotensin Converting Enzyme 2 (ACE2) for entry and the transmembrane serine protease 2 (TMPRSS2) for S protein priming. A TMPRSS2 inhibitor (camostat mesylate, shown below) approved for clinical use has been tested in cell culture and shown to reduce the entry of the virus. This could provide a potential treatment option for patients battling an infection. Additionally from this report, Hoffmann et al., demonstrate that the sera from convalescent SARS patients cross-neutralized SARS-2- S-driven entry. The results from this report provide insight into the commonalities between SARS-CoV-2 and SARS-CoV infection providing a potential target for antiviral intervention.
Additionally, in a separate report, researchers have recently shown that repurposing of an approved drug, hydroxychloroquine phosphate (structure shown below), has been shown to prevent viral infection in vitro (2). In this same report, the nucleoside prodrug remdesivir was administered to a US patient infected with Covid-19 and a beneficial outcome observed. A phase III trial was launched in Wuhan, China on Feb. 4, 2020, but the results and availability of the drug limits its potential utilization in the current pandemic.
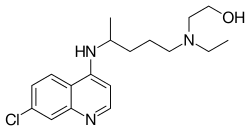
President Trump also announced that this medication would be made available quickly in his daily Covid-19 update press conference on March 19, 2020, but the FDA walked back on this statement stating that a more measured approach is advised for use of hydroxychloroquine.
References
- Hoffmann et al., SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor, Cell (2020), https://doi.org/10.1016/j.cell.2020.02.052
- Liu, J., Cao, R., Xu, M. et al. Hydroxychloroquine, a less toxic derivative of chloroquine, is effective in inhibiting SARS-CoV-2 infection in vitro. Cell Discov 6, 16 (2020). https://doi.org/10.1038/s41421-020-0156-0
- https://www.forbes.com/sites/lisettevoytko/2020/03/19/trump-says-fda-approved-anti-malaria-drug-chloroquine-to-test-as-coronavirus-treatment/#79da412b303d

 中文
中文